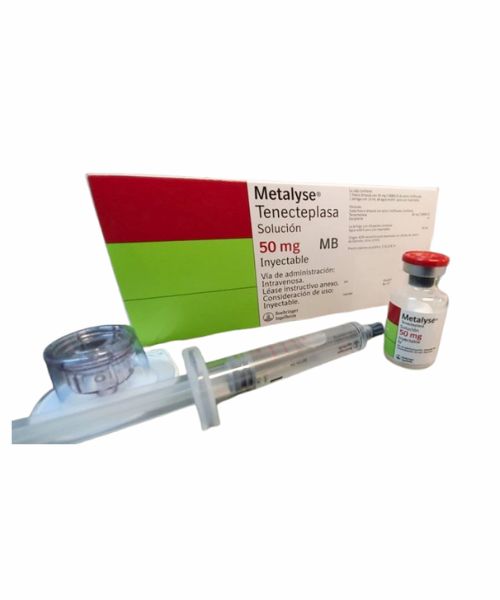
Medicine Guideline of Tenecteplase Metalyse IV 5 mg Injection
Tenecteplase Metalyse IV 5 mg Injection is a adjusted shape of recombinant human tissue plasminogen activator utilized in the crisis treatment of myocardial dead tissue and aspiratory emboli. Metalyse is a Medicine powder and a dissolvable that are made up into a arrangement for infusion. It contains the dynamic substance tenecteplase.
Tenecteplase Metalyse IV 5 mg Injection is a tissue plasminogen activator (tPA) created from alterations of characteristic human tPA complementary DNA (cDNA). It is a 527 amino corrosive with a substitution of threonine 103 with asparagine and substitution of asparagine 117 with glutamine inside the kringle 1 space, and a tetra-alanine substitution at amino acids 296-299 in the protease space.
Tenecteplase Metalyse IV 5 mg Injection is a tissue plasminogen activator (tPA) delivered by recombinant DNA innovation utilizing an set up mammalian cell line (Chinese Hamster Ovary cells). Metalyse is a 527 amino corrosive glycoprotein created by presenting the taking after adjustments to the complementary DNA (cDNA) for characteristic human tPA: a substitution of threonine 103 with asparagine, and a substitution of asparagine 117 with glutamine, both inside the kringle 1 space, and a tetra-alanine substitution at amino acids 296–299 in the protease space.
Tenecteplase Metalyse IV 5 mg Injection is a sterile, white to off-white, lyophilized powder for single intravenous (IV) bolus organization after reconstitution with Sterile Water for Infusion (SWFI), USP. Each vial of Tenectaplase ostensibly contains 52.5 mg Metalyse, 0.55 g L-arginine, 0.17 g phosphoric corrosive, and 4.3 mg polysorbate 20, which incorporates a 5% stuff. Each vial will provide 50 mg of Metalyse.
Tenecteplase Metalyse IV 5 mg Injection Used for
Tenecteplase Metalyse IV 5 mg Injection is utilized to treat grown-ups suspected of having an intense myocardial dead tissue (heart assault) inside six hours of the to begin with indications showing up. It is utilized to break up the blood clots that have shaped interior the blood vessels providing the heart.
Tenecteplase Metalyse IV 5 mg Injection ought to be endorsed by specialists who have encounter in the utilize of thrombolytic medicines (medicines to break up blood clots). Treatment with Metalyse ought to be begun as before long as conceivable after the begin of heart assault side effects. Metalyse is given once as a single infusion into a vein over around 10 seconds. The measurements to be given is balanced concurring to the patient’s weight (see the bundle leaflet).
In expansion to Metalyse, the quiet ought to moreover be treated with other solutions that are utilized to avoid blood clots such as ibuprofen and heparin.
How does Tenecteplase Metalyse IV 5 mg Injection work?
The dynamic substance in Tenecteplase Metalyse, This Injection is a adjusted duplicate of the human protein ‘tissue plasminogen activator’, which the body employments to break down clots. It works by changing over a protein in the clots called plasminogen into its dynamic frame, plasmin, which breaks down the sinewy protein holding the clot together. With the blood clot broken down, blood can stream more effortlessly into the heart muscle, permitting the heart to keep working and making a difference to spare the patient’s life.
Tenecteplase Metalyse IV 5 mg Injection is made by a strategy known as ‘recombinant DNA technology’: it is made by a cell that has gotten a quality (DNA), which makes it able to deliver it.
Side Effects
The most visit antagonistic response related with Metalyse is dying. Ought to genuine dying happen, concomitant heparin and antiplatelet treatment ought to be ceased. Passing or lasting incapacity can happen in patients who encounter stroke or genuine dying scenes. For Tenecteplase Metalyse-treated patients in ASSENT-2, the rate of intracranial hemorrhage was 0.9% and any stroke was 1.8%. The frequency of all strokes, counting intracranial dying, increments with expanding age
Indications
Tenecteplase Metalyse IV 5 mg Injection is shown for utilize in the lessening of mortality related with intense myocardial localized necrosis (AMI). Treatment ought to be started as before long as conceivable after the onset of intense myocardial localized necrosis symptoms.
Pharmacology
Tenecteplase Metalyse IV 5 mg Injection is a adjusted frame of human tissue plasminogen activator (tPA) that ties to fibrin and changes over plasminogen to plasmin. In the nearness of fibrin, in vitro ponders illustrate that Tenecteplase change of plasminogen to plasmin is expanded relative to its transformation in the nonappearance of fibrin. This fibrin specificity diminishes systemic enactment of plasminogen and the coming about debasement of circulating fibrinogen as compared to a particle missing this property.
Taking after organization of 30, 40, or 50 mg of Tenecteplase, there are diminishes in circulating fibrinogen (4%-15%) and plasminogen (11%-24%). The clinical importance of fibrin specificity on security (e.g., dying) or viability has not been set up. Natural power is decided by an in vitro clot lysis measure and is communicated in Tenecteplase-specific units. The particular movement of Tenecteplase Metalyse has been characterized as 200 units/mg.
Dosage
Tenecteplase Metalyse IV 5 mg Injection is for intravenous organization as it were. The prescribed add up to dosage ought to not surpass 50 mg and is based upon understanding weight. A single bolus measurements ought to be managed over 5 seconds based on quiet weight. Treatment ought to be started as before long as conceivable after the onset of AMI symptoms.
Patient Weight <60 kg: 30 mg Tenecteplase
Patient Weight ≥60 to <70 kg: 35 mg Tenecteplase
Patient Weight ≥70 to <80 kg: 40 mg Tenecteplase
Patient Weight ≥80 to <90 kg: 45 mg Tenecteplase
Patient Weight ≥90 kg: 50 mg Tenecteplase
Administration
The item ought to be outwardly assessed earlier to organization for particulate matter and discoloration. Tenecteplase may be managed as reconstituted at 5 mg/mL.
Precipitation may happen when Tenecteplase Metalyse is managed in an IV line containing dextrose. Dextrose-containing lines ought to be flushed with a saline-containing arrangement earlier to and taking after single bolus organization of Tenecteplase.
Reconstituted Tenecteplase ought to be managed as a single IV bolus over 5 seconds. Because Tenecteplase contains no antibacterial additives, it ought to be reconstituted promptly some time recently utilize. If the reconstituted Tenecteplase is not utilized promptly, refrigerate the Tenecteplase vial at 2-8°C and utilize inside 8 hours.
Although the provided syringe is consistent with a customary needle, this syringe is outlined to be utilized with needleless IV frameworks. From the data underneath, take after the enlightening appropriate to the IV framework in use.
Interaction
Formal interaction thinks about of Tenecteplase Metalyse IV 5 mg Injection with other drugs have not been performed. Patients considered in clinical trials of Metalyse were routinely treated with heparin and ibuprofen. Anticoagulants (such as heparin and vitamin K opponents) and drugs that change platelet work. (such as acetylsalicylic corrosive, dipyridamole, and GP IIb/IIIa inhibitors) may increment the hazard of dying if managed earlier to, amid, or after Tenectaplase therapy.
Contraindications
Tenecteplase Metalyse IV 5 mg Injection treatment in patients with intense myocardial dead tissue is contraindicated in the taking after circumstances since of an expanded hazard of bleeding:
- Active inside bleeding
- History of cerebrovascular accident
- Intracranial or intraspinal surgery or injury inside 2 months
- Intracranial neoplasm, arteriovenous deformity, or aneurysm
- Known dying diathesis
- Severe uncontrolled hypertension
Pregnancy & Lactation
Pregnancy: Tenecteplase Metalyse has been appeared to inspire maternal and developing life harmfulness in rabbits given different IV organizations. In rabbits managed 0.5, 1.5, and 5.0 mg/kg/day amid organogenesis, vaginal hemorrhage come about in maternal passings. Ensuing embryonic passings were auxiliary to maternal hemorrhage and no fetal peculiarities were watched.
Tenectaplase does not evoke maternal and developing life harmfulness in rabbits taking after a single IV organization. In this way, in formative poisonous quality thinks about conducted in rabbits, the no perceptible impact level (NOEL) of a single IV organization of Tenectaplase on maternal or formative harmfulness (5 mg/kg) was roughly 7 times human presentation (based on AUC) at the dosage for AMI. There are no satisfactory and well controlled ponders in pregnant ladies. Tenectaplase ought to be given to pregnant ladies as it were if the potential benefits legitimize the potential chance to the fetus.
Nursing Moms: It is not known if Tenecteplase Metalyse is excreted in human drain. Since numerous drugs are excreted in human drain, caution ought to be worked out when Tenectaplase is managed to a nursing woman.
Precautions & Warnings
General: Standard administration of myocardial localized necrosis ought to be executed concomitantly with Tenecteplase Metalyse treatment. Blood vessel and venous punctures ought to be minimized. Noncompressible blood vessel cut must be dodged and inner jugular and subclavian venous punctures ought to be maintained a strategic distance from to minimize dying from the noncompressible locales. In the occasion of genuine dying, heparin and antiplatelet specialists ought to be ceased instantly. Heparin impacts can be switched by protamine.
Readministration: Readministration of plasminogen activators, counting Metalyse, to patients who have gotten earlier plasminogen activator treatment has not been efficiently examined. Three of 487 patients tried for counter acting agent arrangement to Metalyse had a positive counter acting agent titer at 30 days.
The information reflect the rate of patients whose test comes about were considered positive for antibodies to Metalyse in a radioimmunoprecipitation test, and are exceedingly subordinate on the affectability and specificity of the measure. Moreover, the watched frequency of counter acting agent inspiration in an measure may be affected by a few variables counting test dealing with, concomitant drugs, and basic illness.
For these reasons, comparison of the rate of antibodies to Tenecteplase Metalyse with the rate of antibodies to other items may be deluding. In spite of the fact that maintained counter acting agent arrangement in patients accepting one measurements of Metalyse has not been archived, readministration ought to be embraced with caution.
Hypersensitivity: Extreme touchiness, counting urticarial / anaphylactic responses, have been detailed after organization of Metalyse (e.g., anaphylaxis, angioedema, laryngeal edema, hasty, and urticaria). Screen patients treated with Metalyse amid and for a few hours after mixture. If indications of touchiness happen, fitting treatment ought to be initiated.
Use in Special Populations
Pediatric Utilize: The security and viability of Tenecteplase Metalyse in pediatric patients have not been established.
In elderly patients: The benefits of Metalyse on mortality ought to be carefully weighed against the hazard of expanded unfavorable occasions, counting bleeding.
Storage Conditions
Store lyophilized Tenecteplase Metalyse IV 5 mg Injection at controlled room temperature not to surpass 30°C or beneath refrigeration 2-8°C. Do not utilize past the termination date stamped on the vial.




Reviews
There are no reviews yet.